Introduction
Women’s health has traditionally been assumed as equivalent to that of men. However, research has confirmed that health outcomes for many diseases are different for women. The differing biology of women poses very different types of risks as well as presentation of diseases, as compared to their male counterparts[1]. This presents both challenges and opportunities for development of novel platforms for women’s health. Devices and mobile applications are being developed, especially for women to address their health issues. In this context, there is an emerging industry that offers women-centric healthcare solutions based on their fundamental physiological and genetic gender differences, the market for which is predicted to be a $50 billion industry by 2025.
What is Femtech
Femtech or ‘female technology’ encompasses diagnostics, feminine products and wearables that comprise devices and mobile apps. The apps also come with evidence-based recommendations offering holistic female health including fertility and pregnancy-related issues[2].
From adolescence to old age, females need different types of care during each phase. Femtech aims at dissemination of the required knowledge as well as appropriate solutions at each stage[3]. The balanced cyclical life of women represents three unique phases: Maiden, Mother and Crone, each representing specific challenges[4].
A) Women-centric problems / issues
a) Maiden stage:
The challenges in the maiden stage revolve around sexual health, including puberty, menstrual health, sexually transmitted infections and other conditions such as endometriosis, fibroids or polycystic ovarian syndrome.



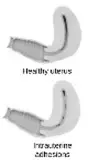 Womed Leaf’s intrauterine adhesion barrier film offers protection against adhesions to the entire uterine cavity for a week. It is inserted through the cervix with a flexible inserter, just like an IUD, at the end of the surgical procedure. The uterine film is not sticky and the insertion takes less than 1 minute, which after a week, breaks-up, dissolves and is naturally discharged through the cervix without any intervention of a healthcare professional.[13]
Womed Leaf’s intrauterine adhesion barrier film offers protection against adhesions to the entire uterine cavity for a week. It is inserted through the cervix with a flexible inserter, just like an IUD, at the end of the surgical procedure. The uterine film is not sticky and the insertion takes less than 1 minute, which after a week, breaks-up, dissolves and is naturally discharged through the cervix without any intervention of a healthcare professional.[13] Madorra’s device is aimed at alleviating symptoms of vulvovaginal atrophy (VVA), a condition that results from a decrease in the body’s estrogen levels. Used at the vaginal opening (not inserted), the device applies therapeutic ultrasound waves along the vaginal canal to stimulate local heat and blood flow, producing natural vaginal lubrication, which researchers claim will alleviate symptoms of VVA[14].
Madorra’s device is aimed at alleviating symptoms of vulvovaginal atrophy (VVA), a condition that results from a decrease in the body’s estrogen levels. Used at the vaginal opening (not inserted), the device applies therapeutic ultrasound waves along the vaginal canal to stimulate local heat and blood flow, producing natural vaginal lubrication, which researchers claim will alleviate symptoms of VVA[14]. MYELLE™ Vaginal Rejuvenation Wand has nineteen red LED lights (650nm), out of which, three are located on the wand’s tip. These red LED lights (650nm) have a regenerative effect on the cells, and can enhance muscle repair, increase elasticity and promote cell turnover, creating healthier, younger skin. This allows for maximum exposure, and the freedom to insert the device is based on the user’s comfort level. Sonic vibrations stimulate the muscles of the pelvic floor, resulting in contraction and strengthening[15].
MYELLE™ Vaginal Rejuvenation Wand has nineteen red LED lights (650nm), out of which, three are located on the wand’s tip. These red LED lights (650nm) have a regenerative effect on the cells, and can enhance muscle repair, increase elasticity and promote cell turnover, creating healthier, younger skin. This allows for maximum exposure, and the freedom to insert the device is based on the user’s comfort level. Sonic vibrations stimulate the muscles of the pelvic floor, resulting in contraction and strengthening[15].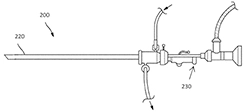 Cytuity: Boston Scientific acquired women’s health-focused NVision Medical, which manufactures a FDA-cleared device designed to collect cells from fallopian tubes as an early diagnosis of ovarian cancer. nVision Medical developed the MAKO 7 device, which is used through the working channel of a hysteroscope for cell collection from the fallopian tube. nVision Medical was acquired by the Boston Scientific Corporation in 2018 and the MAKO 7 is now called Cytuity™[16].
Cytuity: Boston Scientific acquired women’s health-focused NVision Medical, which manufactures a FDA-cleared device designed to collect cells from fallopian tubes as an early diagnosis of ovarian cancer. nVision Medical developed the MAKO 7 device, which is used through the working channel of a hysteroscope for cell collection from the fallopian tube. nVision Medical was acquired by the Boston Scientific Corporation in 2018 and the MAKO 7 is now called Cytuity™[16].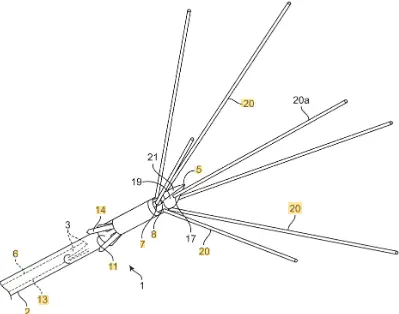
 Clearblue® Advanced Digital Ovulation Test works differently from other ovulation tests as it is designed to detect two hormones, estrogen and Luteinizing hormone (luteinizing hormone). It tracks these two fertility hormones to identify high and peak fertility days, by which the woman can plan ahead and have more opportunities to get pregnant [23].
Clearblue® Advanced Digital Ovulation Test works differently from other ovulation tests as it is designed to detect two hormones, estrogen and Luteinizing hormone (luteinizing hormone). It tracks these two fertility hormones to identify high and peak fertility days, by which the woman can plan ahead and have more opportunities to get pregnant [23]. Ava, a wearable fertility tracker, is an FDA-approved femtech device intended to measure and display physiological parameters (body temperature, resting pulse rate, heart rate variability and breathing rate) as an aid in ovulation prediction. It tracks physiological parameters with built-in sensors. The temperature sensor provides information on skin temperature. A photo plethysmography (PPG) sensor measures the inter-beat interval (IBI) providing information on pulse rate (HR), heart rate variability (HRV), breathing rate (BR) and perfusion. Only HR, HRV, and BR are used by the algorithms for prediction of the fertile window. The user synchronizes the bracelet with the mobile app each morning, which provides Trying to Conceive Mode, Cycle Tracking Mode and Pregnancy Mode. The algorithm is run on the physiological parameters collected, thereby predicting the ovulation and the fertile window displayed in the mobile app along with single mean value for HR, temperature, HRV ratio, BR, and sleep duration from the prior night[24].
Ava, a wearable fertility tracker, is an FDA-approved femtech device intended to measure and display physiological parameters (body temperature, resting pulse rate, heart rate variability and breathing rate) as an aid in ovulation prediction. It tracks physiological parameters with built-in sensors. The temperature sensor provides information on skin temperature. A photo plethysmography (PPG) sensor measures the inter-beat interval (IBI) providing information on pulse rate (HR), heart rate variability (HRV), breathing rate (BR) and perfusion. Only HR, HRV, and BR are used by the algorithms for prediction of the fertile window. The user synchronizes the bracelet with the mobile app each morning, which provides Trying to Conceive Mode, Cycle Tracking Mode and Pregnancy Mode. The algorithm is run on the physiological parameters collected, thereby predicting the ovulation and the fertile window displayed in the mobile app along with single mean value for HR, temperature, HRV ratio, BR, and sleep duration from the prior night[24]. Mira: The Mira Fertility tracker monitors menstrual cycles and ovulation by measuring basal body temperature as well as hormonal changes in urine or saliva. It includes an analyzer, which is an oval, pod shaped device that evaluates hormone levels in the urine collected by the dip system, and syncs with the Mira App and uses artificial intelligence to track hormone patterns [27].
Mira: The Mira Fertility tracker monitors menstrual cycles and ovulation by measuring basal body temperature as well as hormonal changes in urine or saliva. It includes an analyzer, which is an oval, pod shaped device that evaluates hormone levels in the urine collected by the dip system, and syncs with the Mira App and uses artificial intelligence to track hormone patterns [27].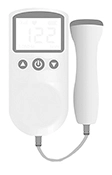 AccuSure Fetal Doppler is a radiation-free Heartbeat Monitor with TFT display. The Fetal Doppler can be utilized after about four months of pregnancy. The device brings out a clear sound of the baby’s heartbeat[28].
AccuSure Fetal Doppler is a radiation-free Heartbeat Monitor with TFT display. The Fetal Doppler can be utilized after about four months of pregnancy. The device brings out a clear sound of the baby’s heartbeat[28]. Elvie Pump: A wireless, wearable pump that fits directly on to breasts, Elvie Pump features a motor located within the breast cup itself. The breast pump tells how much milk is produced and has a mechanism to stop automatically while preventing leaks. Elvie Pump is a silent wearable breast pump, making it possible for new mothers to pump anytime, anywhere[32]. This product is protected by US11357894B2.
Elvie Pump: A wireless, wearable pump that fits directly on to breasts, Elvie Pump features a motor located within the breast cup itself. The breast pump tells how much milk is produced and has a mechanism to stop automatically while preventing leaks. Elvie Pump is a silent wearable breast pump, making it possible for new mothers to pump anytime, anywhere[32]. This product is protected by US11357894B2.